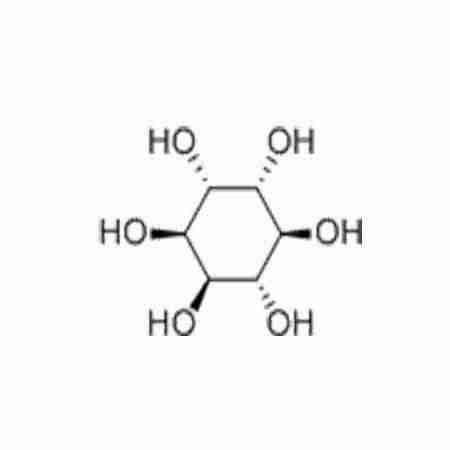
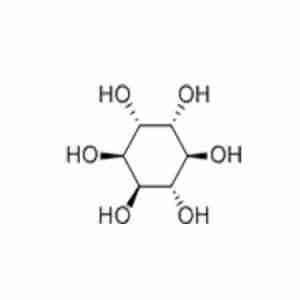
D-CHIRO-INOSITOLTypical Properties
Items of Analysis
Standard of Analysis
Test Result
Appearance
White powder
White powder
Assay(%,HPLC)
≥95%
96.87%
Other sugars(HPLC)
≤5%
0.10%
Tap Density
≤0.85g/ml
0.805g/ml
Particle Size
90% pass 80mesh
Conforms
Loss on drying
≤5.0%
0.12%
Residue on Ignition
≤5.0%
0.02%
Pb
≤3.0ppm
0.0415ppm
As
≤3.0ppm
0.4627ppm
Cd
≤1.0ppm
0.0046ppm
Hg
≤0.1ppm
0.0003ppm
Total Bacteria,CFU/g
≤1000cfu/g
90cfu/g
Yeasts & Molds,CFU/g
≤100cfu/g
10cfu/g
E.Coli
Negative
Negative
Coli-form bacteria
Negative
Negative
Salmonella
Negative
Negative
Brand name
Fousi chemical
D-CHIRO-INOSITOL Usage
Inositol and its phosphates have been used to develop metabolically stable insulin mediators, inhibitors, and modulators of important metabolic functions such as glycolysis.
Inositol is very stable to degrading enzymes in the body because of the lack of hydrolysis-labile glycosidic bonds.
D-CHIRO-INOSITOL Packaging and Shipping
Packing: 25Kg/drum
D-CHIRO-INOSITOL Storage
It should be placed in cool dry warehouse