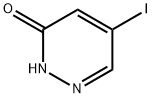
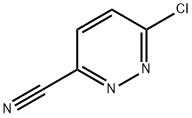
Cobalt sulfate heptahydrate CAS# 10026-24-1
Cobalt sulfate heptahydrate, Promotion Season Now in Store and Free Sample for Testing with Factory Price
- Appearance: Red powder
- Assay: 96.8%min
- Packaging: 25kg/Plastic woven bags.
- Sample: Available
.jpg)




Cobalt sulfate heptahydrate: The Complete Guide
- Chapter 1: Quality Control of Cobalt sulfate heptahydrate
- Chapter 2: Description of Cobalt sulfate heptahydrate
- Chapter 3: Basic Info of Cobalt sulfate heptahydrate
- Chapter 4: What is Cobalt sulfate heptahydrate?
- Chapter 5: What’s the application of Cobalt sulfate heptahydrate?
- Chapter 6: Keywords of Cobalt sulfate heptahydrate
- Chapter 7:WHY THEY SAY TO US
- Chapter 8: Hot Sale Products
Request A Free Quote
Quality Control of Cobalt sulfate heptahydrate
Quality Control & MSDS
Chemical Structure
.jpg)
Packing of Cobalt sulfate heptahydrate


Description of Cobalt sulfate heptahydrate
Cobalt(II) sulfate is any of the inorganic compounds with the formula CoSO4(H2O)x. Usually cobalt sulfate refers to the hydrate CoSO4.7H2O, which is one of the most commonly available salts of cobalt.
Basic Info of Cobalt sulfate heptahydrate
Basic Info
Chemical Name | cobalt(2+) sulfate |
Synonyms | Cobalt(II) sulfate heptahydrate;Cobalt sulfate heptahydrate;cobalt(2+),sulfate,heptahydrate; |
CAS No. | 10026-24-1 |
Molecular Formula | CoH14O11S |
Molecular Weight | 281.10300 |
PSA | 153.25000 |
LogP | -0.70730 |
Safety Info
RTECS | GG3200000 |
Safety Statements | S22-S45-S53-S60-S61 |
HS Code | 28332930 |
Packing Group | III |
WGK Germany | 2 |
RIDADR | UN 3077 |
Risk Statements | R22; R42/43; R49; R50/53 |
Hazard Codes | T; N |
Hazard Declaration | H302; H317; H334; H341; H350i; H360F; H410 |
Caution Statement | P201; P261; P280; P284; P304 + P340; P308 + P313 |
Symbol | GHS07, GHS08, GHS09 |
Signal Word | Danger |
Properties
Appearance & Physical State | Red crystal or crystalline powder |
Density | 2.03 |
Boiling Point | 735ºC |
Melting Point | 98ºC |
Water Solubility | 362 g/L (20 ºC) |
Stability | Stable. Non-flammable. Hygroscopic. Dehydrates at around 41ºC and 71ºC. |
Storage Condition | Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed. |
Numbering system
EINECS number | 233-334-2 |
RTECS number | GG3200000 |
MDL number | MFCD00149658 |
What is Cobalt sulfate heptahydrate?
With the progress of science and technology and the development of society, chemical products have invariably permeated our daily lives, in medicine, food, cosmetics, electronics, industry, and other areas, becoming an essential part of our lives. One such product is Cobalt sulfate heptahydrate which has developed particularly rapidly in recent years. Do you know about Cobalt sulfate heptahydrate?
The official answer:Soluble in water, methanol, slightly soluble in ethanol, easily weathered in air. Dissolved in a mixture of sulfuric acid and nitric acid with metallic cobalt, heated to boiling with steam, and concentrated by evaporation. It is crystallized and separated by cooling. It can also be made by reacting oxidized drill and sulfuric acid. It is used as an additive in the estimation of electric transition liquid, drilling iron magnetic material, paint drying, glaze drug, chemical analysis reagent, catalyst, alkaline battery. Cobalt(II) sulfate heptahydrate is also known as cobalt sulfate, cobalt sulfate, cobalt(II) sulfate. It is a rose-red crystal. After dehydration, it is a red powder.
What’s the application of Cobalt sulfate heptahydrate?
used as ceramic glaze, paint drying agent, also used in dyestuff
Used in cobalt plating solution for electroplating industry. Also used as cobalt-iron magnetic material, paint drying agent, glazing agent for colored porcelain, additive for an alkaline battery, chemical analysis reagent and catalyst, etc.
Inhalation and skin contact may cause allergy and cancer. Highly toxic to aquatic organisms, may have long-term adverse effects on the water environment
Avoid contact, special instructions must be obtained before use. The substance and its container must be disposed of as hazardous waste.
An aqueous solution of sulfuric acid is heated and cobalt trioxide is added. Formaldehyde is then added to it for reduction and the solution gradually turns clear. Filter, evaporate the filtrate until a crystalline film is formed, cool, crystallize and dry to obtain pure cobalt sulfate.
Conclusion
Sincere to the customer and in good faith of quality is our forever followed motto. It’s the basement to be a human and do business. We take all responsibility for our products and service sincerely. We are a company integrating R&D, production, and sales. The design, research, and development of pharmaceutical intermediates including more than 330 kinds of pharmaceutical intermediates such as Anti-infectives, Cardiovascular and Cerebrovascular Systems, Digestive System, Santi-tumor, Nervous System, Geriatric Drugs, and Gynecological Drugs. At the same time, we have more than 4,000 factories in strategic cooperation with nearly 50,000 chemical products, so we can provide you with a one-stop purchasing service, which greatly saves customers’ procurement costs and time.

Hello, I’m Pew Ni, the chemical products one-stop solution expert.
Hi, I‘m Peter Ni, the founder of Sincere-Chem, I’ve been running a company in China that exporting chemicals, pharmaceuticals, and intermediates for over 70 countries now. And a range of 50,000 products with the best quality and competitive price to meet your different purchasing needs.
CEO Email: inquiry@sincerechemical.com
CEO Phone Number:
+86-188-6575 9396
CEO WhatsApp:
+86-188-6575 9396
used as ceramic glaze, paint drying agent, also used in dyestuff
Used in cobalt plating solution for electroplating industry. Also used as cobalt-iron magnetic material, paint drying agent, glazing agent for colored porcelain, additive for an alkaline battery, chemical analysis reagent and catalyst, etc.
Inhalation and skin contact may cause allergy and cancer. Highly toxic to aquatic organisms, may have long-term adverse effects on the water environment
Avoid contact, special instructions must be obtained before use. The substance and its container must be disposed of as hazardous waste.
An aqueous solution of sulfuric acid is heated and cobalt trioxide is added. Formaldehyde is then added to it for reduction and the solution gradually turns clear. Filter, evaporate the filtrate until a crystalline film is formed, cool, crystallize and dry to obtain pure cobalt sulfate.
Request A Free Quote
Keywords of Cobalt sulfate heptahydrate
Cobalt sulfate heptahydrate, 10026-24-1, buy Cobalt sulfate heptahydrate, Cobalt sulfate heptahydrate supplier, purchase Cobalt sulfate heptahydrate, Cobalt sulfate heptahydrate cost, Cobalt sulfate heptahydrate manufacturer, order Cobalt sulfate heptahydrate, high purity Cobalt sulfate heptahydrate.
WHY THEY SAY TO US