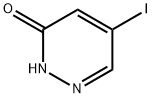
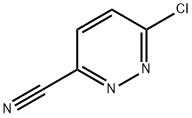
Bretazenil CAS# 84379-13-5




Bretazenil, Promotion Season Now in Store and Free Sample for Testing with Factory Price
- Assay: ≥98%
- Appearance: White powder
- Capacity: 300kg/month
- Packaging: 25 kg/drum, 1kg/foil bag
- Sample: available
Bretazenil: The Complete Guide
- Chapter 1: Quality Control of Bretazenil
- Chapter 2: Description of Bretazenil
- Chapter 3: Basic Info of Bretazenil
- Chapter 4: What is Bretazenil ?
- Chapter 5: Do you want to know other research results about bretazenil ?
- Chapter 6: Conclusion
- Chapter 7: Keywords of Bretazenil
- Chapter 8: Most Helpful Reviews
- Chapter 9: Hot Sale Products
Request A Free Quote
Quality Control of Bretazenil
Quality Control & MSDS
Chemical Structure

Packing of Bretazenil


Description of Bretazenil
Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988.
It is most closely related in structure to the benzodiazepine antagonist flumazenil, although its effects are somewhat different. It is classed as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome.
Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5 GABAA benzodiazepine receptor complexes.
Basic Info of Bretazenil
Basic Info
| Chemical Name | Bretazenil |
| Synonyms | Ro-16-6028; |
| CAS No. | 84379-13-5 |
| Molecular Formula | C19H20BrN3O3 |
| Molecular Weight | 418.28400 |
| PSA | 64.43000 |
| LogP | 3.81870 |
Numbering system
| UNII | OSZ0E9DGOJ |
Properties
| Density | 1.56g/cm3 |
| Boiling Point | 594.3ºC at 760 mmHg |
| Flash Point | 313.2ºC |
| Refractive Index | 1.685 |
| Storage Condition | 2-8ºC |
Safety Info
| Safety Statements | S22; S24/25 |
What is Bretazenil?
Depression is the most common form of depressive disorder, characterized by significant and persistent depression, and is the main type of mood disorder.
The depressed mood can range from sullenness to grief, low self-esteem and depression, and even pessimism and anxiety, and may include suicide attempts or behaviors; and even wood stiffness; in some cases, marked anxiety and motor agitation; and in severe cases, psychotic symptoms such as hallucinations and delusions. So there are many antidepressants on the market, one of the products is bretazenil which is good for antidepressants, do you know what bretazenil is?
Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family and was invented in 1988. It is most closely related in structure to the benzodiazepine antagonist flumazenil, although its effects are somewhat different.
It is classed as a high-potency benzodiazepine due to its high-affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile.
In particular, bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5, and α6 subunit-containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5 GABAA benzodiazepine receptor complexes.
Do you want to know other research results about bretazenil?
Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil.
The use of benzodiazepines (BDZs) in the long-term treatment of epilepsy is greatly restricted by their capacity to induce tolerance and dependence. Thus, the development of new BDZ-related therapeutic agents should be directed by strategies that minimize tolerance- and dependence-inducing properties.
Experimental procedures used to determine the success of such strategies often rely on a single assay procedure (e.g., one seizure model), which might lead to false predictions. Furthermore, the different types of tolerance, i.e., “pharmacological” (metabolic or functional) and “behavioral” (“learned” or “contingent”), are often not dealt with in such studies.
This prompted us to compare the chronic anticonvulsant efficacy and withdrawal characteristics of diazepam and two novel BDZ receptor ligands, i.e., the partial agonist Bretazenil and the subtype-selective agonist abecarnil, in different seizure models in mice. Myoclonic, clonic, and tonic seizures were induced by i.v. infusion of pentylenetetrazol and by transcorneal or transauricular application of electrical stimuli. Prolonged administration of diazepam (5 mg/kg twice daily for 6 days) resulted in marked anticonvulsant effects on myoclonic, clonic, and tonic seizure thresholds at the onset of treatment, but pronounced tolerance developed rapidly during subsequent treatment. The time course and extent of tolerance were similar to most seizure models. Tolerance characteristics were not affected by study design, i.e., the use of separate or the same animals for each seizure induction, indicating that learned or contingent tolerance was not significantly involved under these experimental conditions.
After termination of treatment with diazepam, significant seizure threshold decreases were determined, indicating withdrawal hyperexcitability in response to physical dependence. During prolonged administration of abecarnil (10 mg/kg twice daily for 6 days), some anticonvulsant tolerance was seen with electroshock seizures, but not with pentylenetetrazol seizures; no withdrawal hyperexcitability was determined upon the termination of treatment. Bretazenil (10 mg/kg twice daily for 6 days) produced no tolerance in any of the seizure models, but a significant decrease in electroshock seizure threshold was seen in the withdrawal period.
The data indicate that tolerance and withdrawal characteristics of BDZ receptor partial and subtype-selective agonists in mice depend on the experimental model used, whereas the influence of the experimental protocol is less critical in the case of a full BDZ receptor agonist such as diazepam.
Conclusion
Sincere Chemical has been dedicated to the research and development of antidepressants and the reform of production processes for more than ten years and has achieved a number of continuous, large-scale, and intelligent manufacturing technologies for antidepressants and related fine chemicals with independent intellectual property rights.
With more than 10 automated production plants, the company produces hundreds of products in a wide range of industries, including more than 330 kinds of pharmaceutical intermediates such as Anti-infectives, Cardiovascular and Cerebrovascular Systems, Digestive systems, Santi-tumor, Nervous systems, Geriatric Drugs, and Gynecological Drugs. We welcome inquiries about our products and technical knowledge of bretazenil.

Hello, I’m Pew Ni, the chemical products one-stop solution expert.
Hi, I‘m Peter Ni, the founder of Sincere-Chem, I’ve been running a company in China that exporting chemicals, pharmaceuticals, and intermediates for over 70 countries now. And a range of 50,000 products with the best quality and competitive price to meet your different purchasing needs.
CEO Email: inquiry@sincerechemical.com
CEO Phone Number:
+86-188-6575 9396
CEO WhatsApp:
+86-188-6575 9396
Request A Free Quote
Keywords of Bretazenil
Most Helpful Reviews
Hot Sale Products